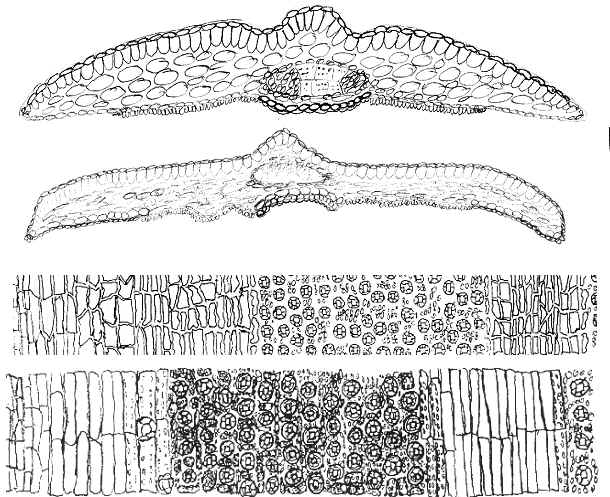
|
The
Taxus
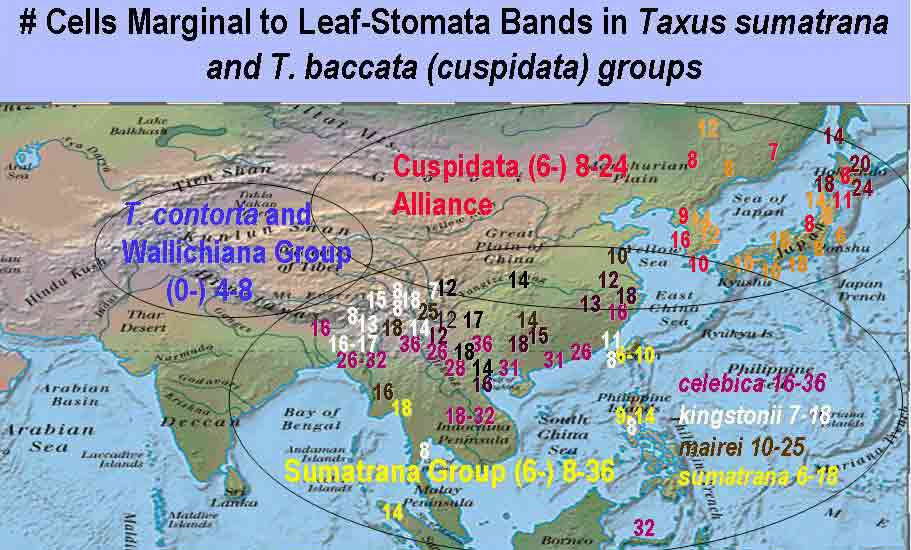
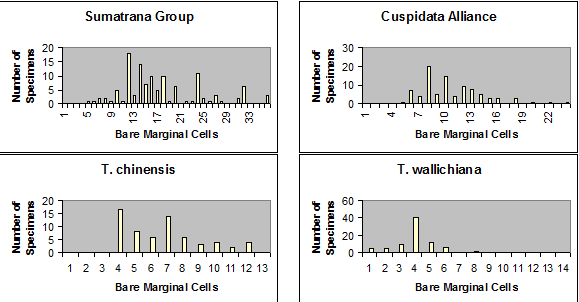
Sumatrana Group is characterized by leaves having a relatively broad
region of marginal cells to the stomata bands, varying from 8–36
cells across, further differentiated by the irregular shape of epidermal
cells and by their discoloration upon drying, appearing glossy
and reddish when dried—as shown below. Leaves in other
species groups usually have stomata bands bordered by fewer marginal cells, or
marginal cells are less differentiated from stomata bands in shape, color, and
in their development of papillae. The above figure excludes the
Subgroup
Chinensis to emphasize the contrast among the three major
groups of Taxus in Asia. The Chinensis subgroup is
depicted on a similar map for number of stomata rows per band under the
Wallichiana Group,
and also in the following figure for number of marginal cells, from Spjut
(2007a); the vertical axis shows the number of specimens tabulated for
the number of marginal cells across the abaxial leaf margin
(without papillae) indicated along the horizontal axis (see illustrated
examples above).
The species
and varietal taxonomy of the Sumatrana
Group is difficult due to partly overlapping character
traits among taxa. Previous
taxonomists recognized only one taxon in this group, either as a
variety (Cheng & Fu 1975, 1978; Li & Fu 1997), or species
(Florin 1948; Handel Manzzetti 1929; Hu 1964), but
the
correct name was not applied. De Laubenfels
(1978) for instance regarded
T.
sumatrana as the name for all yew plants in Southeast Asia
(East Himalayas to Sumatera), but this is antedated by
Taxus wallichiana. This broad
species concept
(T. wallichiana) makes it difficult
to distinguish T. wallichiana from
Taxus cuspidata and its allies (Taxus baccata Group).
Spjut (2007b,
2013, this website)
distinguishes a Sumatrana Group of four species and three
varieties, two varieties remain unpublished. The taxa are differentiated by
leaf characters that include three aspects of leaf curvature: (1) lengthwise
vertically (down-curved vs. up-curved
near apex), (2) lengthwise horizontally as evident by shape such as machete-shaped (curved more on one side
than the other), sickle-shaped (curved equally along both sides) vs. straight linear to oblong
for most of their length, and (3) transverse (convex vs. flat) across the adaxial leaf surface.
These characters are partially correlated with the leaf tapering
from its widest point, and whether they taper symmetrically or asymmetrically to
base and to apex. Leaves shortly tapered to apex may have parallel margins for most
the the leaf length as seen in
T. mairei var. mairei, a leaf shape
also referred to as
oblong except for its asymmetrical tapered base and apex, the overall
shape generally machete-like.
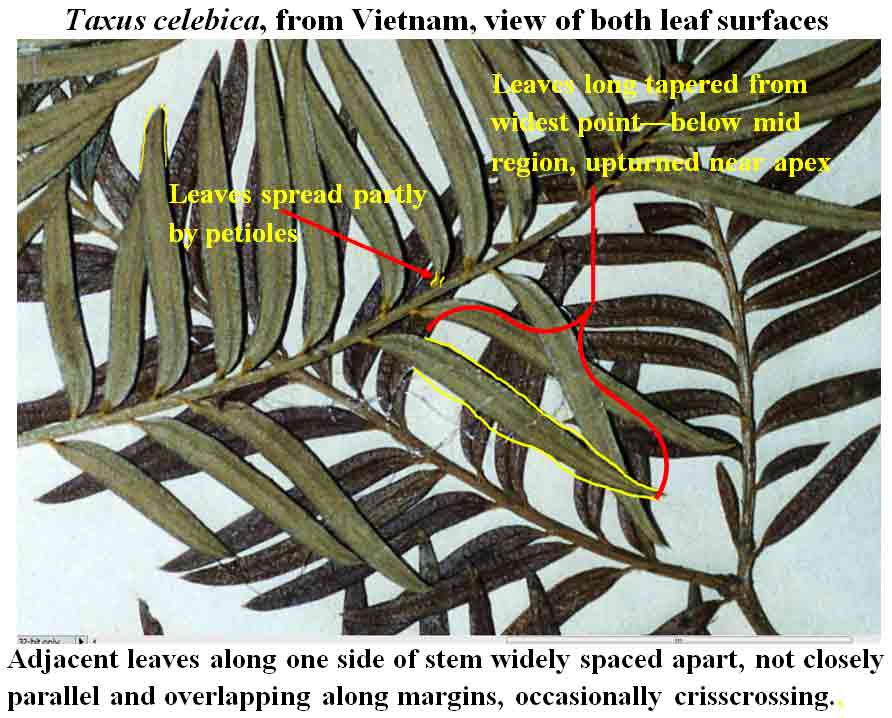
Taxus
celebica and
T. sumatrana leaves taper
to apex starting from well below the mid region of the blade,
and except for appearing arcuate near base, their overall shape is
lanceolate (sword-shaped). Leaves of
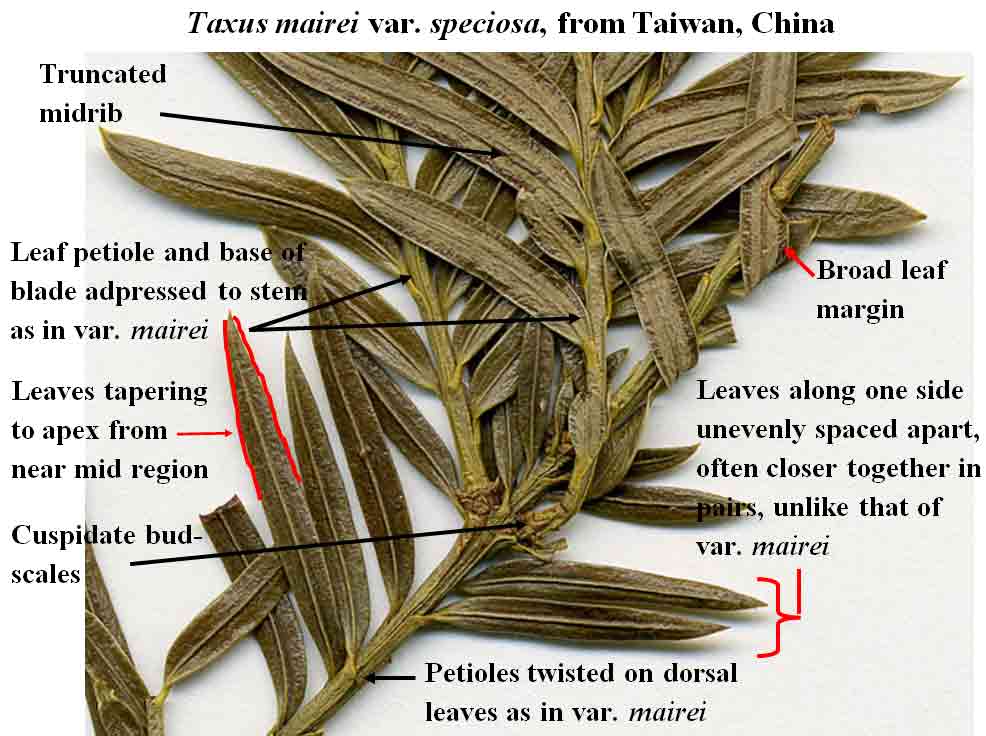
T. mairei
var. speciosa often taper to apex from near the mid region, but their
shape is not always elliptical since its leaves are often strongly curved along both
sides, appearing sickle shaped.
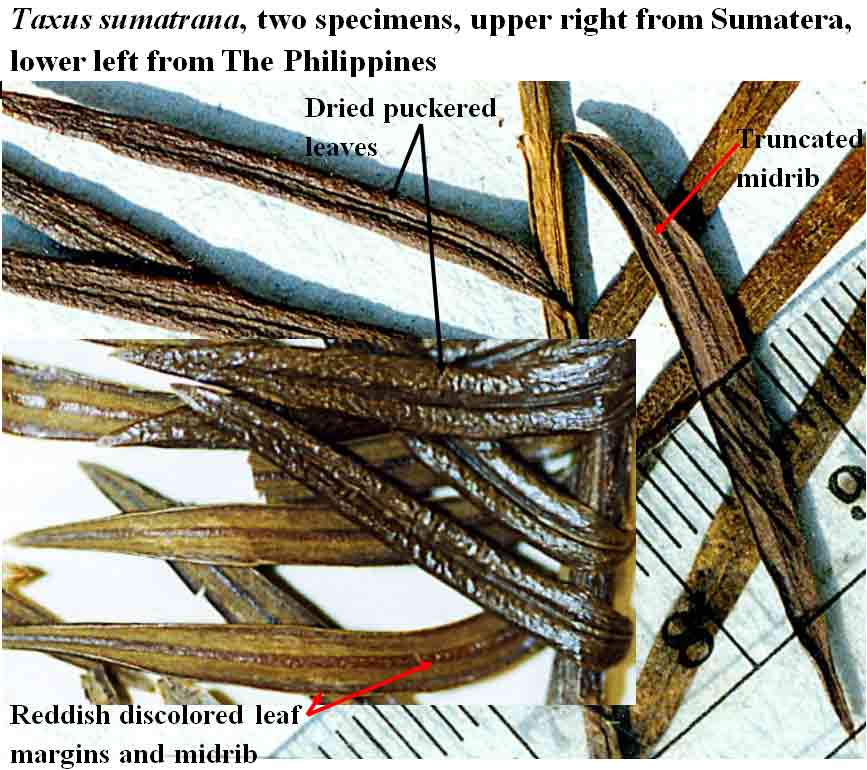
The abaxial leaf midribs of T. sumatrana and T.
mairei are elevated with truncated margins and a central channel.
The epidermal cells are usually inflated (mammillose). This is in
contrast to a keeled midrib of non-inflated epidermal cells in T. kingstonii,
in which its midrib may vary on a plant from an elevated round keel to
nearly flush
with the leaf surface.
The species
are also differentiated by the dried leaves, particularly the color of stomata
bands and puckering of the blade surface to the extent that channels
appear along each
side of the adaxial midrib (see T. sumatrana below).
The degree to
which leaves spread from a branch is partly related to the habit of the
plant and its habitat. A yew growing on an open rocky slope most
likely will have ascending to erect branches from which leaves may not
spread out as far as one growing in the forest understory that will
likely have more horizontal branches and divergent leaves. It is
important to keep in mind that leaves in all species of Taxus
generally spread to expose the abaxial surface away from direct light,
but the manner in which this is done varies.
Species are
also distinguished by the frequency of leaf
development
along a branch—as may be measured by distance
between adjacent leaves (phyllotaxy). The spiral arrangement of leaves
follows a reoccurring pattern (phyllotaxy) along a branch in which leaves
reappear along one line, and those along that line may spread differently from those along other
lines on the same branch. Their arrangement may be further affected by
whether they spread by bending of the petiole
and/or by bending or twisting of the blade. These differences appear partially correlated
with leaf shape. Treating these characters independently
in multivariate analyses (Möller et al 2013) may lead to biased results.
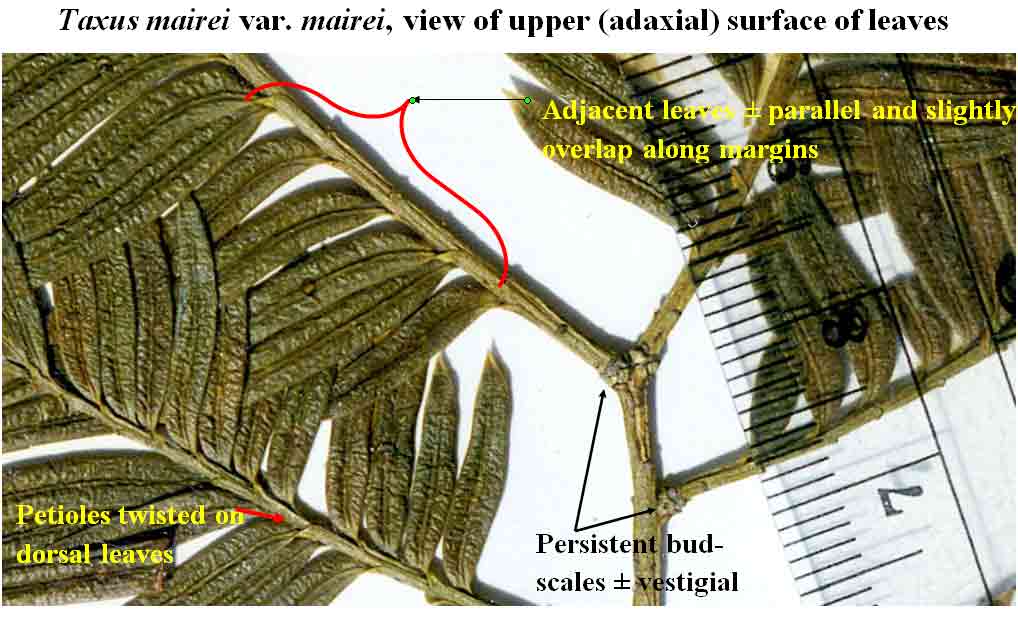
An easy
to recognize phyllotaxy is that of
Taxus mairei
var. mairei. In many plants, leaves develop at 1 mm intervals
with a complete cycle at 6 mm as measured from the junction of blade
with petiole. Its leaf arrangement is also distinctive in that the leaves spread out from the stems by
a
sharp twist of the blade near base where they, along with their
petioles, are adpressed to the stem, rather
than spreading from branches strictly by the petiole. It may be noted that leaves
of
Taxus mairei
var. mairei developing on the underside (ventral
surface) of stems
sharply bend, while those developing more along the dorsal surface
sharply twist, a
common trait to most species of Taxus. Not only do the
leaves of T. mairei var. mairei align in one plane along two sides of a branch, but they appear
almost consistently equidistant and parallel to one another.
However,
phyllotaxy and leaf arrangement in related species are not as
distinctive; for example,
Taxus celebica
from Sulawesi (type) has larger leaves slightly more separated
from one another, developing at ~3 mm intervals with a completed cycle at
22 mm, but not all spread in the same direction along one side of the branch; some
leaves diverge more by bending of the petioles with their blades appearing relatively
straight, while other leaves are arcuate near base.
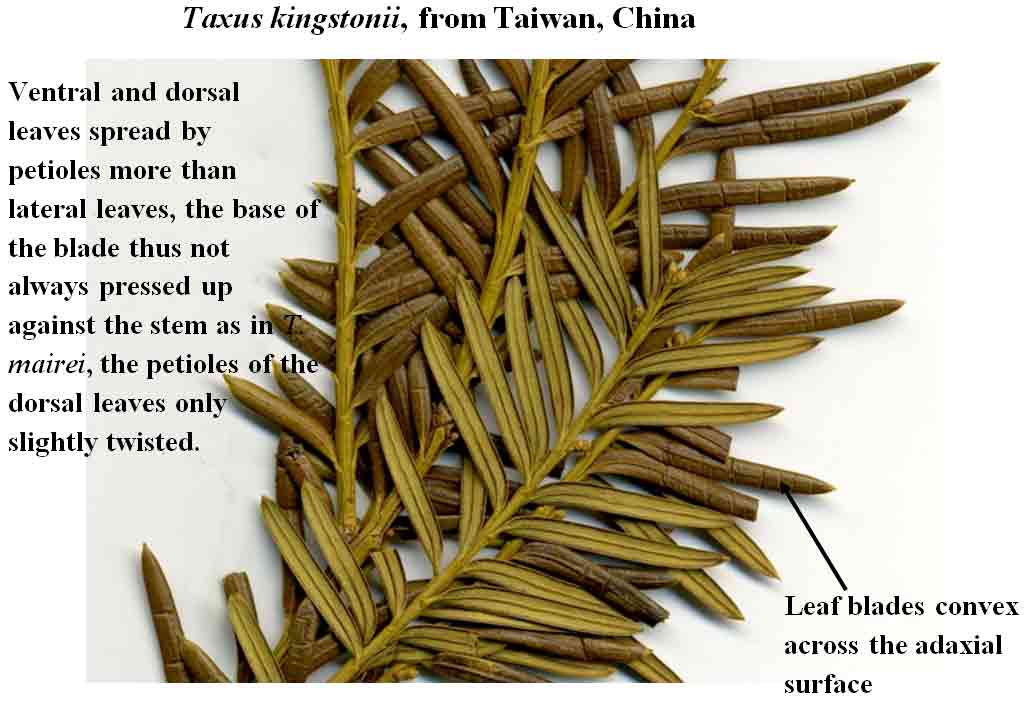
Taxus kingstonii
in Taiwan has leaves developing at 4 mm intervals with a completed cycle
at 14 mm, and they crisscross more
frequently as seen along one side of the branch, a phyllotaxy
intermediate between that of T. celebica and T. mairei var.
mairei. This type of spread seems related to machete shape of
the leaves, in contrast to more
linear, symmetrically tapered leaves of T. kingstonii that spread
away from the branch more by its petiole.
Species
variation in phyllotaxy has not been quantitatively assessed,
because other factors such as leaf shape and size and branch thickness
appear correlated, and that genetic differences within a species are also
evident. Nevertheless, phyllotaxy is largely distinctive for
distinguishing most
species and varieties of Taxus.
Generally, each species within the Taxus sumatrana group is
recognized by a suite of character features from which one or more
are given more taxonomic weight for identification, while the same taxonomic
character states do not apply to distinguishing all species in the
Taxus sumatrana Group. For example, phyllotaxy
and the lanceolate acuminate leaf shape are considered most important
for Taxus celebica, while also appearing correlated with dried leaves retaining a green
color on the adaxial surface and remaining flat across both surfaces. Taxus sumatrana
has similarly shaped but slightly smaller leaves, but taxonomic weight
is given to its leaves that—upon drying—become puckered across the
adaxial surface and recurved
along margins, while also turning dark green
to blackish green in color. Taxus mairei
and its var. speciosa—treated as varieties because of their close similarity in leaf anatomy and
geographical distribution—are distinguished by many features that
include phyllotaxy, leaf arrangement, leaf shape, branching pattern, and seed color
and seed shape; taxonomic weight is subjectively applied mostly in that order. Their
morphological differences and close geographical association
(Spjut 2007b) appears correlated with molecular data (Gao et
al. 2007, haplotypes #13,
#14), but their treatment as varieties by Spjut
(2007b) is contrary to Möller et al (2013) who argued
that they should not be distinguished unless geographically
distinct. Taxonomic weight for recognizing Taxus kingstonii is
given to its rusty
orange colored stomata bands in dried leaves, and to its leaves curving
downwards lengthwise along the blade, sometimes more strongly near leaf tips, and
to the blades
not adpressed to stem at base. This is in sharp
contrast to the upturned leaves of T. mairei var. mairei
where they are tapered to apex and are adpressed up against the stem at base.
In putative hybrids from northern Yunnan (T. celebica, T.
mairei var. speciosa, T. kingstonii), the pale orange
leaf color was given the most weight for identifying T. kingstonii.
Examples of Specimens for Species in the
Taxus sumatrana Group
-
Taxus
kingstonii—What to look for in leaves:
1. Spreading at various
angles from branchlets (stems) more by bending of the petiole than
by curving of the blade.
2. Appearing convex across the adaxial surface.
3. Curved downwards lengthwise along the blade, sometimes
with a slight twist, especially where they taper to apex.
4. ± Symmetrically tapered to apex
and to base.
5. Stomata bands relatively broad, in contrast to a narrow
region of marginal cells, often discolored rusty orange in herbarium
specimens.
6. Abaxial midrib
elevated into a round keel or flush with the surface as sometimes seen on a
single leaf, occasionally truncated at margins in putative hybrids with
T. mairei var. speciosa or T. celebica.
|
|
Möller et al (2013) carved out a “hidden"
"new species” they recognized to occur from Yunnan to Vietnam, distinguished by the absence of
papillae of the abaxial leaf midrib and by bud-scales persisting at the
base of branchlets as
related to their determination of one of 19 chloroplast haplotypes (Gao
et al. 2007),
clip of illustration of type shown below from Möller et al (2013).
The variation in the papillae on the abaxial midrib was discussed in
detail by Spjut (2003) under
Taxus chinensis.
The lack of
papillae on the abaxial midrib suggests that Hengduan Mountain species
in question may belong to the Sumatrana Group; however, judging
from the phyllotaxy and leaf shape in the illustration (Möller et al
2013), it would appear to belong to the Chinensis Subgroup (Spjut
2007b). The use of the papillae character should be
corroborated by other characters such as leaf epidermal cell shape in
cross section and number of abaxial leaf marginal cells without papillae (Spjut 2007a,b),
characters that Möller et al (2013) excluded from their study, in which
they employed principal component analysis (PCA) for 27 morphological
characters based on Möller et al (2007), except for modification to how leaves
are inserted.
A major flaw with
morphometric analyses is that biological species distributions are not
linear; rather, they are geometric (Willis 1922). One might expect
character features to cluster for the common species, a biased result in
which data for large numbers of individuals sampled randomly may appear
convincing when visualized on graphs; however, there is likely to a
black hole in the data in considering the "hollow curve" distribution
(See also: Spjut, 2010; Review of plants collected for antitumor
screening, Fig. 1, p. 18).
http://www.worldbotanical.com/images/ARS%20NCI%20Active%20Plants/Review%20of%20plants%20collectod%20for%20antitumor%20screening-Spjut-2010.pdf.
The rare species may appear as anomalies. The results are further
biased when one holds the view that species must be geographically
separated.
The
persistence of bud-scales can be variable in T. chinensis in that
they can persist longer at the base of some branches more than others as evident in
this link to a specimen from
Hubei. Among the
species in the Sumatrana Group, T. kingstonii is
most similar to T. chinensis. Spjut (2007b) noted that he
reidentified his annotations for some specimens of T. chinensis—that were initially
identified from examination under a dissecting scope—to T. kingstonii
when leaf sections were later prepared and examined under a compound microscope.
Thus, from his point of view the distinction of Möller et al (2013) new species is
questionable. Taxus scutata Spjut ineditus is also similar
to the illustration but would seem to differ by having larger
(conspicuous persistent) bud scales and a papillose abaxial midrib
(Spjut 2007b, 2013; also this website under the Chinensis
Subgroup).
Further, it
may noted that Möller et al (2013) limited their study to mainland China
with reference to their earlier studies on Himalayan yew plants (Möller et
al 2007). They
excluded specimens from Taiwan, while they also recognize T. mairei
to occur outside China such as in northeastern India, where specimens
studied by Spjut (2007a,b) from that region were identified as
belonging to either T. celebica, T. kingstonii, or T.
sumatrana, but not to T. mairei, which Spjut (2007) considers
endemic to southern China, corresponding to Gao et al. (2007)
distribution of Taxus haplotyptes #13, #14. It should be
noted that T.
celebica and T. sumatrana are earlier names for T. mairei
that Möller et al (2013) failed to account for in their paper,
possibly because the types come from Indonesia and not China.
The other species
of the Sumatrana Group appear to occur less frequently in mainland China (Spjut 2007a,b), thus,
such rare occurrences may not stand out in PCA, or possibly occur as
rare haplotypes in Gao et al. (2007). Additionally, Möller et
al (2013) suggest that the disjunct occurrences of T. kingstonii
have discordant character features that belong to T. mairei,
whereas Spjut (2007b) proposed that the variation he saw in T.
kingstonii may be
due to hybridization with
T. wallichiana
in northeastern India, with
T. chinensis
and
T. mairei
in Shaanzi, Gansu and Sichuan,
and with T. celebica
in Yunnan.
Both Spjut
(2007a) and Möller et al (2013) recognize hybridization between T.
wallichiana and T. chinensis on Mount Emei based on different
leaf characters and different analytical methods, while Möller et
al (2013) did not reference Spjut's (2007a) study. But
their philosophical views on species differ. Möller et al (2013)
reportedly look for a geographical fit to their data, drawing on
geographical defined their preconceived species (e.g., T. mairei occurring from India
to China while excluding T. sumatrana), whereas Spjut (2007a,b) defined species based on
reoccurring morphological character traits regardless of their geographical
continuity or discontinuity (Spjut 2000, 2007, 2013). Nonetheless, Spjut's (2007)
character features show phytogeographical relationships
for defining species groups, species subgroups and the species, which
all are supported by
molecular data from other studies (J. Li 2001; Gao et al. 2007; Hao et
al. 2008a,b; Shah et al. 2008), and included morphological characters that were not
employed by Möller et al (2013). And while Möller et al (2013) may
see Spjut's species concept of Taxus as narrow,
many disjunct taxa recognized by Spjut (2007b, 2013) in reality reflect a broad
species concept by their wide geographical distribution; he (Spjut 2007b) had also stated that his objective was to recognize
the fewest species and varieties that could be consistently defined
based on key subjectively correlated character features as noted by Möller et
al (2013).
Key to Species and Varieties
in the Taxus sumatrana Group
References:
see Introduction to Taxus,
Overview of the genus Taxus, and Spjut
2007a, 2007b.
Spjut, R. W.
2007a. A phytogeographical analysis of Taxus (Taxaceae) based on
leaf anatomical characters. J. Bot. Res. Inst. Texas 1(1): 291–332
Spjut, R. W. 2007b.
Taxonomy and nomenclature of Taxus. J. Bot. Res. Inst. Texas
1(1): 203–289.
Willis, J. C. 1922.
Age and area. Cambridge University Press.
|